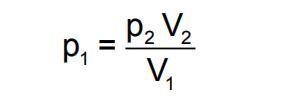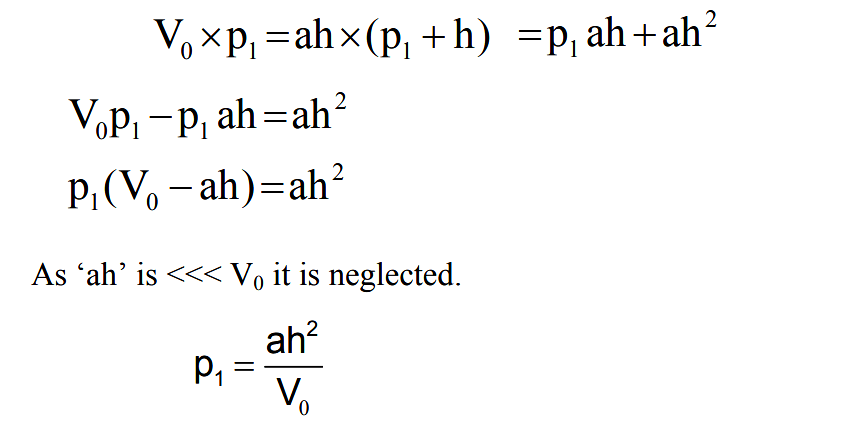Measurement of vacuum by two methods :
1. Direct measurement
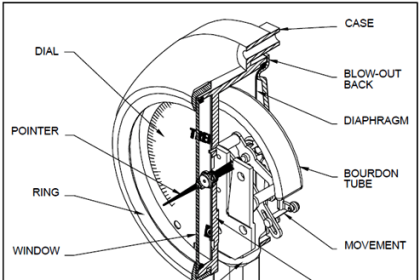
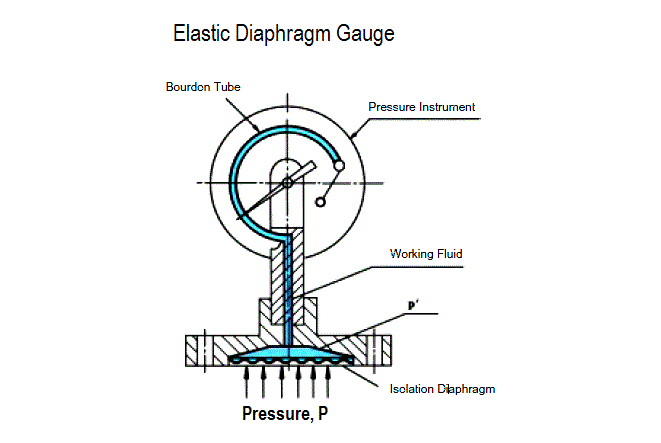
Resulting in a displacement caused by the action of force [Spiral Bourdon tubes, flat or corrugated diaphragm, capsules and various other manometers]
2. Indirect measurement or inferential methods
Pressure is determined through the measurement of certain pressure controlled properties such as volume, thermal conductivity etc.
McLeod Gauge Vacuum Gauge is a inferential method of measuring vacuum
McLeod gauge
The working of McLeod Gauge is based on Boyles‟ fundamental equation.
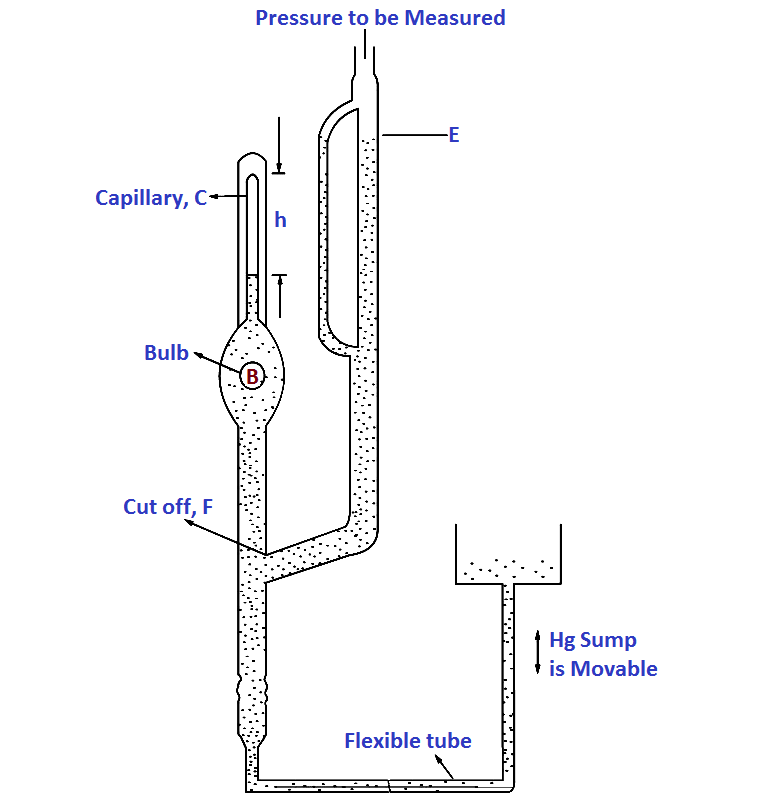
where p and V refer to pressure and volume respectively and subscripts 1 and 2 refer to initial and final conditions. Conventional McLeod gauge is made of glass. Refer Below Fig, It consists of the capillary „C‟, bulb „B‟ and the mercury sump which is connected to the lower end of the glass tube such that it can be moved up and down.
The pressure to be measured (the unknown pressure) is connected to the upper end of the glass part. When the mercury level in the gauge is below the cut off „F‟, the unknown pressure fills the gauge including the bulb B and capillary C.
When the mercury sump is moved up, the level in the gauge rises and when it reaches the cut off „F‟ a known volume of gas at pressure to be measured is trapped in bulb B and capillary C.
Mercury is then forced up into the bulb and capillary. Assume the sump is raised to such a level that the gas at the pressure to be measured which filled the volume above the cut off is now compressed to the volume represented by the column h. Suppose the original volume after then mercury reaches F is V0 . This is at a pressure being measured p1
Applications of McLeod gauge
McLeod gauge is used mainly for calibrating other inferential type of gauges. The shortcomings of the McLeod gauge are its fragility and the inability to measure continuously. The vapor pressure of Mercury sets the lower limit of measurement range of the gauge.
Advantages of the McLeod Gauge:
- It is independent of the gas composition.
- It serves as a reference standard to calibrate other low pressure gauges.
- A linear relationship exists between the applied pressure and h
- There is no need to apply corrections to the McLeod Gauge readings.
Limitations of McLeod Gauge:
- The gas whose pressure is to be measured should obey the Boyle’s law
- Moisture traps must be provided to avoid any considerable vapor into the gauge.
- It measure only on a sampling basis.
- It cannot give a continuous output.