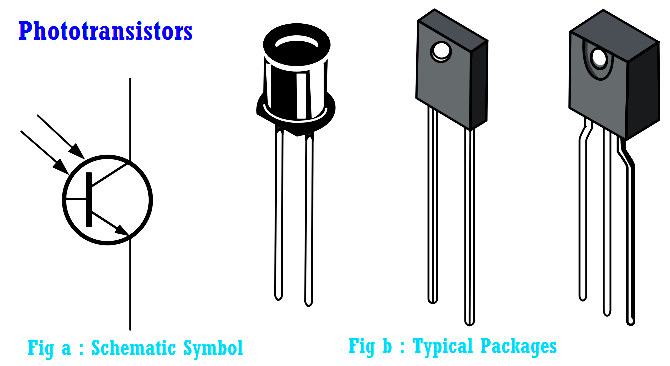
Phototransistor Working Principle
A phototransistor is similar to a regular BJT except that the base current is produced and controlled by light instead of a voltage source. The phototransistor effectively converts light energy to…
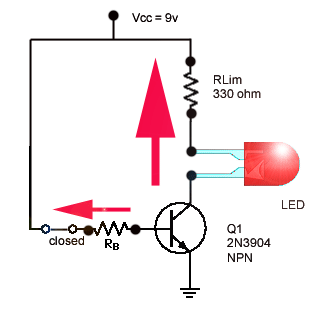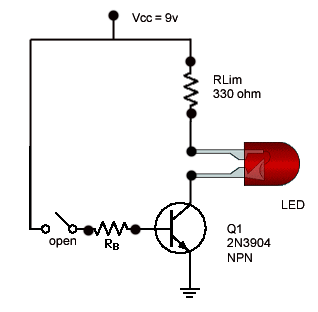
How a Transistor Switch Works
The transistor in Figure is used as a switch to turn the LED on and off. For example, a square wave input voltage with a period of 2 s is applied…
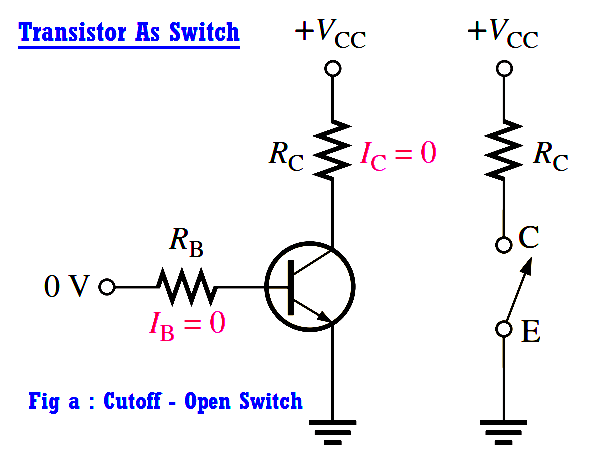
Transistor as a Switch Working Principle
The below Figure illustrates the basic operation of a BJT as a switching device. In part (a), the transistor is in the cutoff region because the base-emitter junction is not forward-biased.…
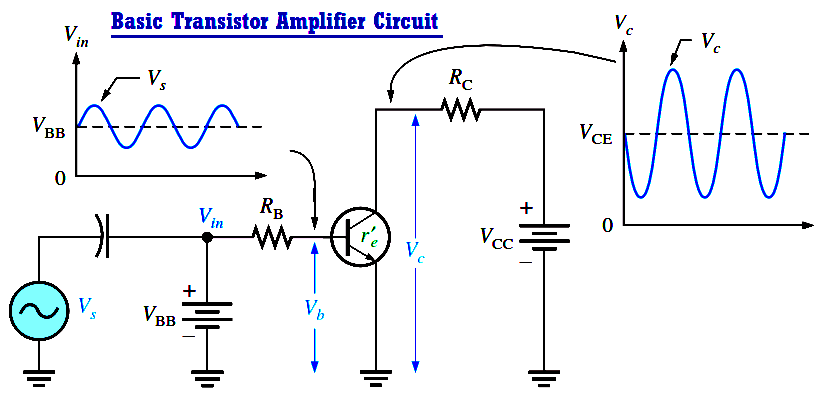
Basic Transistor Amplifier Circuit Principle
A transistor amplifies current because the collector current is equal to the base current multiplied by the current gain, b. The base current in a transistor is very small compared to the collector…
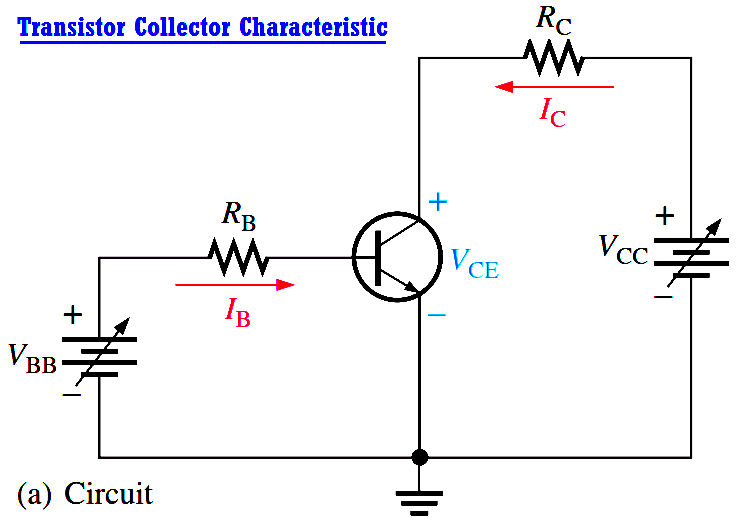
Transistor Collector Characteristic Curves
Using a circuit like that shown in Figure (a), a set of collector characteristic curves can be generated that show how the collector current, IC, varies with the collector-to-emitter voltage, VCE,…
Why Silicon is preferred over Germanium ?
As we all know, both Silicon and Germanium are semiconductor devices. But the present trend is to use Silicon instead of Germanium. What may be the reasons? Although both silicon…
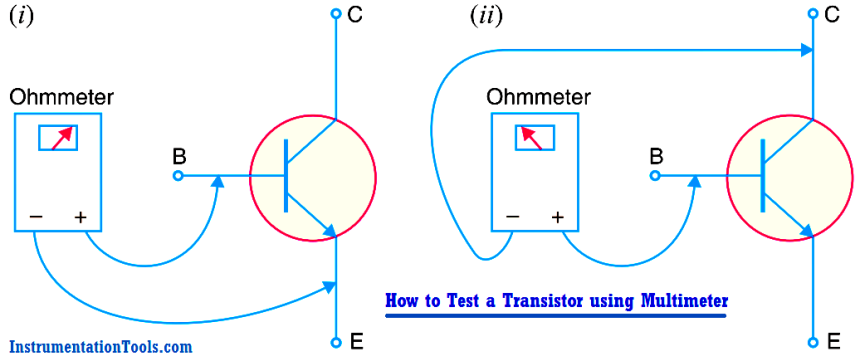
How to Test a Transistor using Multimeter
An ohmmeter can be used to check the state of a transistor i.e., whether the transistor is good or not. We know that base-emitter junction of a transistor is forward…
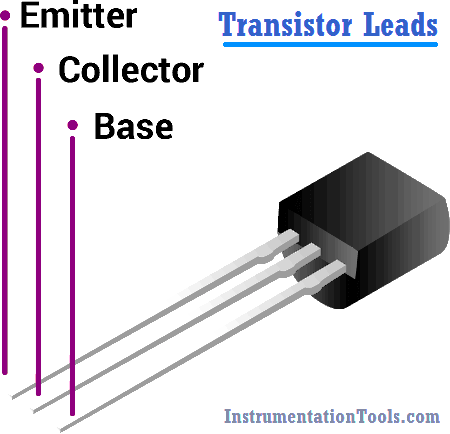
How to Identify the Transistor Terminals
There are three leads in a transistor viz. collector, emitter and base. When a transistor is to be connected in a circuit, it is necessary to know which terminal is…
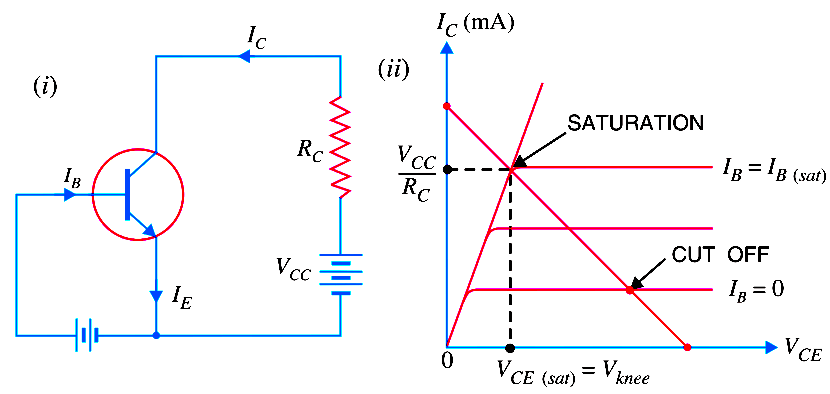
Transistor Cut off, Saturation & Active Regions
The below Fig. (i) shows CE transistor circuit while Fig.(ii) shows the output characteristcs along with the d.c. load line. (i) Cut off. The point where the load line intersects…
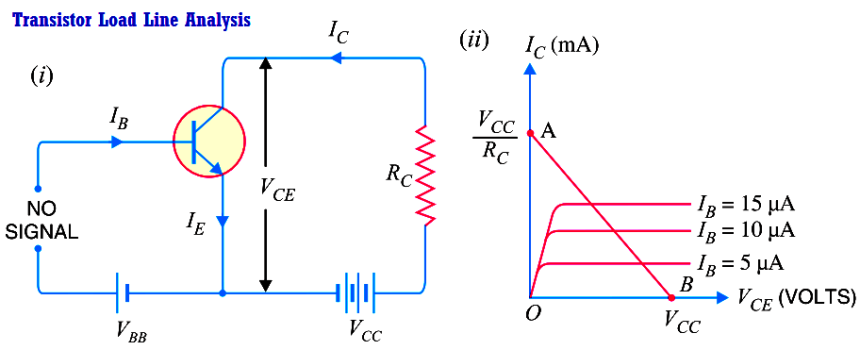
Transistor Load Line Analysis
In the transistor circuit analysis, it is generally required to determine the collector current for various collector-emitter voltages. One of the methods can be used to plot the output characteristics…