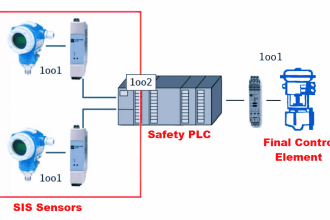
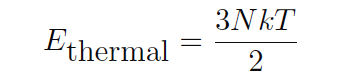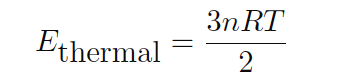Temperature is the measure of average molecular kinetic energy within a substance. The concept is easiest to understand for gases under low pressure, where gas molecules randomly shuffle about. The average kinetic (motional) energy of these gas molecules defines temperature for that quantity of gas. There is even a formula expressing the relationship between average kinetic energy (Ek) and temperature (T) for a monatomic (single-atom molecule) gas:
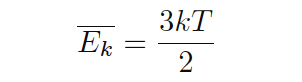
Where,
Ek = Average kinetic energy of the gas molecules (joules)
k = Boltzmann’s constant (1.38 × 10−23 joules/Kelvin)
T = Absolute temperature of gas (Kelvin)
Thermal energy is a different concept: the quantity of total kinetic energy for this random molecular motion. If the average kinetic energy is defined as 3kT/2 , then the total kinetic energy for all the molecules in a monatomic gas must be this quantity times the total number of molecules (N) in the gas sample:
This may be equivalently expressed in terms of the number of moles of gas rather than the number of molecules (a staggeringly large number for any realistic sample):
Where,
Ethermal = Total thermal energy for a gas sample (joules)
n = Quantity of gas in the sample (moles)
R = Ideal gas constant (8.315 joules per mole-Kelvin)
T = Absolute temperature of gas (Kelvin)
Heat is defined as the exchange of thermal energy from one sample to another, by way of conduction (direct contact), convection (transfer via a moving fluid), or radiation (emitted energy); although you will often find the terms thermal energy and heat used interchangeably.
Illustrating by way of example, a single molecule of gas moving at a constant velocity will have a definite temperature. Two or three molecules moving at the same speed will have the same temperature, but together represent a greater thermal energy than any one of them considered alone. Heat is either the reduction or increase of thermal energy by transfer of energy. If these gas molecules happen to collide with slower-moving gas molecules, the faster molecules will lose velocity while the slower molecules will gain velocity. Thus, the higher-temperature molecules cool down while the lower-temperature molecules warm up: a transfer of heat.
Temperature is a more easily detected quantity than either thermal energy or heat. In fact, when we need to measure either thermal energy or heat, we do so by measuring temperature and then inferring the desired variable based on the laws of thermodynamics.
There are many different ways to measure temperature, from a simple glass-bulb mercury thermometer to sophisticated infrared optical sensor systems. Like all other areas of measurement, there is no single technology that is best for all applications. Each temperature-measurement technique has its own strengths and weaknesses. One responsibility of the instrument technician is to know these pros and cons so as to choose the best technology for the application, and this knowledge is best obtained through understanding the operational principles of each technology.