Standard temperature and pressure conditions as well as normal temperature and pressure conditions are used as reference points in thermodynamics of gases. Normally standard and normal temperature pressure conditions are used for specifying vapor volume.
This is because, volume of a given number of vapor moles is a function of temperature and pressure conditions. Hence it becomes imperative to specify the corresponding temperature and pressure conditions for volume measurement, whenever gas quantity is specified in terms of gas volume.
Hence universally recognized reference temperature and pressure conditions can be easily used to specify gas volume measured at those conditions. This gas volume can be readily converted to number of moles or gas mass, as temperature and pressure of this standard reference point is easily known.
Values of standard temperature and pressure depend on the organization which defines them. Usually the standard pressure is close to atmospheric pressure and standard temperature is close to room temperature value.
Standard conditions for temperature and pressure are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions.
In chemistry, IUPAC has changed the definition of standard temperature and pressure (STP) in 1982:
- Until 1982, STP was defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 1 atm (1.01325 × 105 Pa).
- Since 1982, STP is defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 105 Pa (100 kPa, 1 bar).
STP should not be confused with the standard state commonly used in thermodynamic evaluations of the Gibbs energy of a reaction.
NIST uses a temperature of 20 °C (293.15 K, 68 °F) and an absolute pressure of 1 atm (14.696 psi, 101.325 kPa). This standard is also called normal temperature and pressure (abbreviated as NTP).
The International Standard Metric Conditions for natural gas and similar fluids are 288.15 K (15.00 °C; 59.00 °F) and 101.325 kPa.
In industry and commerce, standard conditions for temperature and pressure are often necessary to define the standard reference conditions to express the volumes of gases and liquids and related quantities such as the rate of volumetric flow (the volumes of gases vary significantly with temperature and pressure). However, many technical publications (books, journals, advertisements for equipment and machinery) simply state “standard conditions” without specifying them, often leading to confusion and errors. Good practice always incorporates the reference conditions of temperature and pressure.
Past use
Before 1918, many professionals and scientists using the metric system of units defined the standard reference conditions of temperature and pressure for expressing gas volumes as being 15 °C (288.15 K; 59.00 °F) and 101.325 kPa (1.00 atm; 760 Torr). During those same years, the most commonly used standard reference conditions for people using the imperial or U.S. customary systems was 60 °F (15.56 °C; 288.71 K) and 14.696 psi (1 atm) because it was almost universally used by the oil and gas industries worldwide. The above definitions are no longer the most commonly used in either system of units.
Current use
Many different definitions of standard reference conditions are currently being used by organizations all over the world. The table below lists a few of them, but there are more. Some of these organizations used other standards in the past. For example, IUPAC has, since 1982, defined standard reference conditions as being 0 °C and 100 kPa (1 bar), in contrast to its old standard of 0 °C and 101.325 kPa (1 atm).
Natural gas companies in Europe, Australia, and South America have adopted 15 °C (59 °F) and 101.325 kPa (14.696 psi) as their standard gas volume reference conditions. Also, the International Organization for Standardization (ISO), the United States Environmental Protection Agency (EPA) and National Institute of Standards and Technology (NIST) each have more than one definition of standard reference conditions in their various standards and regulations.
Table : Standard reference conditions in current use
Table 1 :
| Defining Organization | Temperature in 0C | Pressure in kPa |
| IUPAC | 0 | 100.0 |
| NIST, ISO 10780 | 0 | 101.325 |
| ISA, ISO 13443 | 15 | 101.325 |
| EPA | 25 | 101.325 |
| SATP | 25 | 100.0 |
| CAG | 20 | 100.0 |
| SPE | 15 | 100.0 |
| ISO 5011 | 20 | 101.3 |
Table 2 :
| Defining Organization | Temperature in 0F | Pressure in psi |
| SPE, OSHA | 60 | 14.696 |
| OPEC, U.S. EIA | 60 | 14.73 |
| U.S. Army Standard Metro | 59 | 14.503 |
| ISO 2314, ISO 3977-2 | 59 | 14.696 |
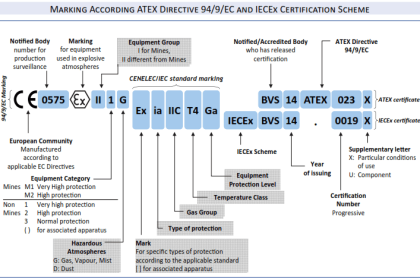
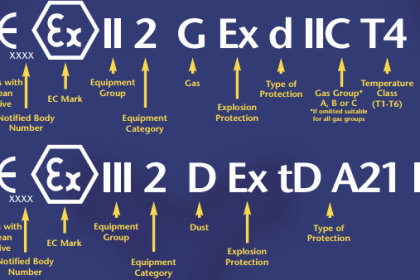



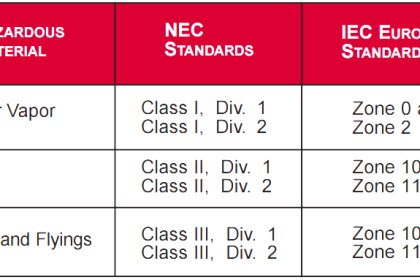




Since standard and normal temperature and pressure conditions don’t have an universally accepted definition up to now, so reference pressure and temperature shall always be specified at the initial phase of project. Normally, such information can be found from the project specification – Unit of Measurement.