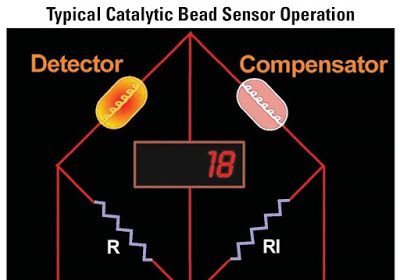
Catalytic-type gas sensor consist of two elements: a detector element (D) which contains catalytic material and is sensitive to combustible gases, and a compensator element (C) which is inert.
Combustible gases will burn only on the detector element, causing a rise in its temperature and, as a consequence, a rise in its resistance.
Combustible gases will not burn on the compensator—its temperature and resistance remain unchanged in the presence of combustible gases. ( below Figure)
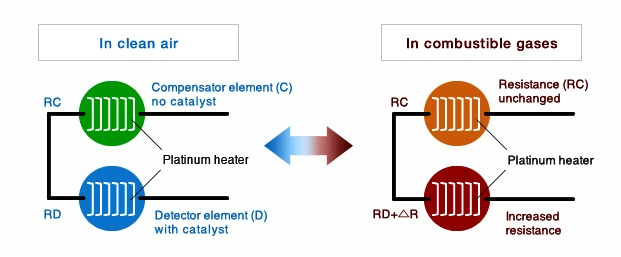
Image Credits : figaro
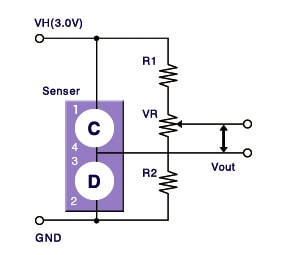
Normally a Wheatstone bridge circuit is formed with both elements as shown in below Figure.
A variable resistor (VR) is adjusted to maintain a state of balance of the bridge circuit in clean air free of combustible gases.
When combustible gases are present, only the resistance of the detector element will rise, causing an imbalance in the bridge circuit, thus producing an output voltage signal (Vout).
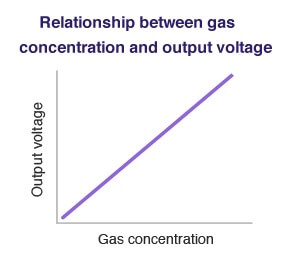
The output voltage signal is proportional to the concentration of combustible gases as shown in below Figure.
Gas concentration can be determined by measuring the output voltage.
Also Read : Why LEL important in Gas Detection?
Credits : figaro