Electrons and protons are the two basic subatomic elements of an atom. Today in this article we will compare both electrons and protons.
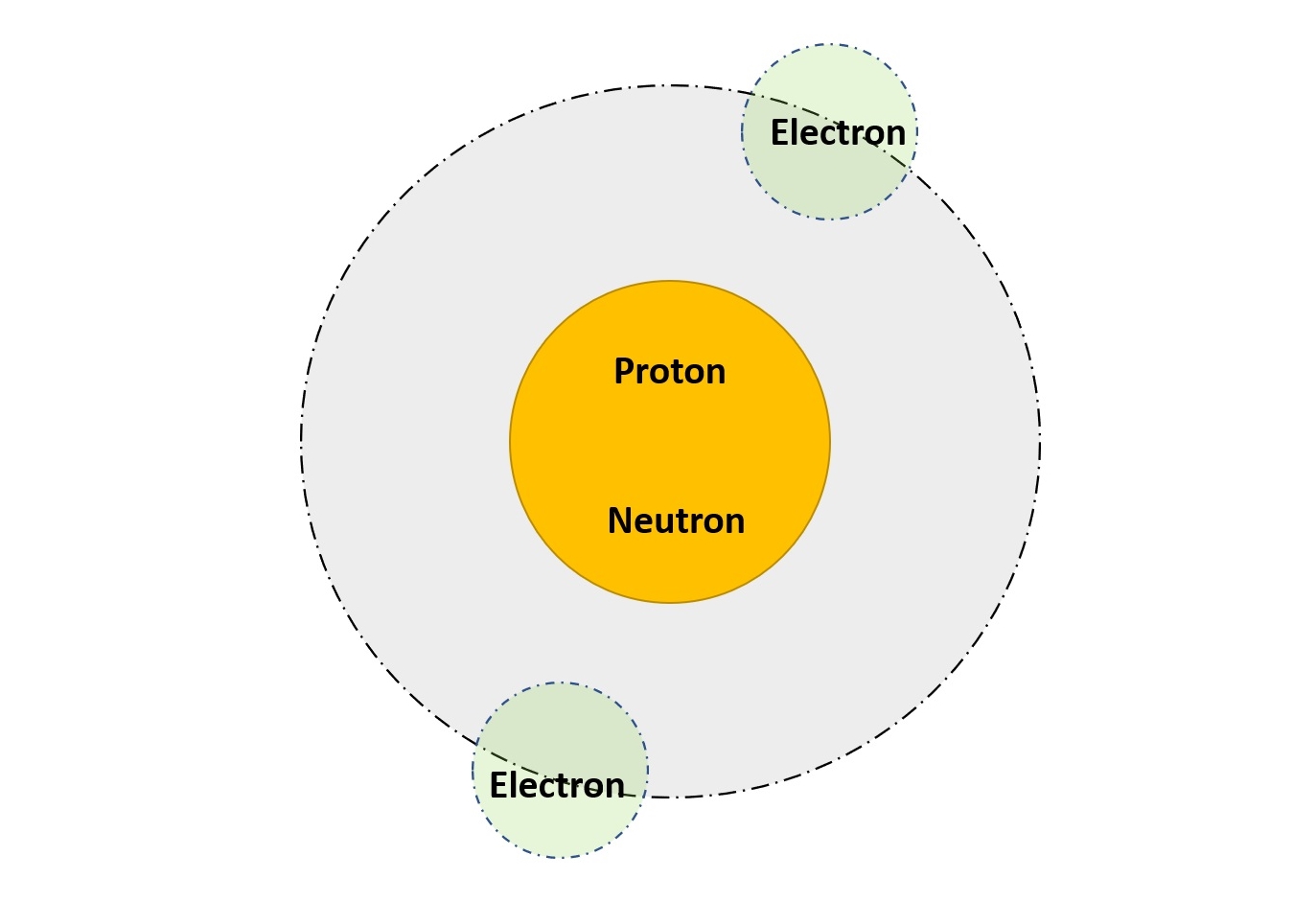
Electrons and protons are basic subatomic elements that form an atom. An atom consists of mainly 2 parts. One is the nucleus of the atom and the other is the extra-nucleus of the atom. Let us look into the detail of electrons and protons.
What is an Electron?
Electrons are the subatomic particle of an atom that are negatively charged in nature. The charge of an electron is -1.6 * 10-19 Coulomb. The mass of an electron is 9.1 * 10-31 kg.
In any atom, the electrons are present in the extra nucleus of the atom. The electrons are continuously revolving in the extra-nucleus of the atom around the nucleus of the atom in an orbit. The name electricity comes from the word electrons. The electron flow in a conductor is considered electricity.
As electrons are present in the space around the nucleus, they can be easily separated from the atom. To separate an electron from an atom, some external energy is needed which will detach the electron from the atom. Because of this process of removal or addition of electrons, the overall charge of an atom changes.
What is a Proton?
Proton is also a subatomic particle of an atom. The proton is a positively charged subatomic particle in nature. The charge of a proton is +1.6 * 10-19 Coulomb. The mass of the proton is 1.67 * 10-27 kg. This means that the proton is much heavier than the electron. The proton is present in the nucleus of the atom.
The presence of the proton in the nucleus makes it positively charged. The protons are present in the nucleus of the atom. Hence, it is very difficult to remove the protons from an atom. Also, it is interesting to know that the total number of protons in an atom decides the atomic number of that element.
Difference between Electron and Proton
Now, let us see some of the key differences between electron and proton:
| Electron | Proton |
| Electron is a negatively charged subatomic particle | Proton is a positively charged subatomic particle |
| The charge of an electron is -1.6 * 10-19 Coulomb | The charge of a proton is +1.6 * 10-19 Coulomb |
| Electron revolves around the nucleus of the atom | Proton is present in the nucleus of the atom |
| The mass of an electron is very less compared to that of the proton | The mass of the proton is very high compared to that of the electron |
| The mass of the electron is 9.1 * 10-31 kg | The mass of the proton is 1.67 * 10-27 kg |
| Electron is denoted by the symbol ‘e’ | Proton is denoted by the symbol ‘p’ |
| Electrons can be easily removed from an atom | Protons cannot be moved out easily from an atom |
| The chemical reactions occurring are due to the presence of electrons only | Protons do not participate in the chemical reactions |
| The number of electrons present in an atom is not taken into consideration when deciding the atomic number | The number of protons present in an atom is the atomic number of that element. |