Sulphur Di-oxide Monitoring Questions & Answers
1. In West-Gaeke colourimetric procedure, the intensity of the red purple colour is measured photometically and it is proportional to the concentration of sulphur di-oxide. What is the red purple coloured compound?
a) Sodium tetra chloromercurate
b) Dicholorosulphitomercurate complex
c) Ammonia molybdate
d) Paraosaniline sulphonic acid
Answer: d
Explanation: Sulphur di-oxide reacts with Sodium tetra chloromercurate to form Dicholorosulphitomercurate complex which then reacts with formaldehyde and pararosaniline to form Parapsaniline sulphonic acid which is red purple coloured.
2. The conductivitimetry method, involves bubbling sulphur di-oxide through a solution containing sulphuric acid and which of the following?
a) Water
b) Hydrogen peroxide
c) Iodine
d) Formaldehyde
Answer: b
Explanation: The conductivitimetry method, involves bubbling sulphur di-oxide through a solution containing sulphuric acid and hydrogen peroxide. It results in the formation of sulphuric acid.
3. Which of the following are not the characteristics of conductivitimetry method used to measure sulphur di-oxide?
a) Fast response
b) High sensitivity
c) Free from interference by other gases
d) Good accuracy
Answer: c
Explanation: Conductivitimetry instruments are affected by other gases and they cause interference. The other gases may produce or remove ions in the solution.
4. Which of the following are not the characteristics of colorimetry method used to monitor sulphur di-oxide?
a) It is simple
b) It has high sensitivity
c) It has good specificity
d) It is affected by interference from H2SO4, SO3, NH3, etc
Answer: d
Explanation: Colorimetry method is free from by the interference due to other gases such as It is affected by interference from H2SO4, SO3, NH3, etc. Colour intensity is sensitive to temperature.
5. Which of the following is not true about using gas chromatography for measuring pollutants in air?
a) Pollutants react rapidly in the column
b) Pollutants may not be detected by the detectors
c) Special column and support materials are required
d) Pollutants may elude rapidly from the column
Answer: d
Explanation: Most of the pollutants are extreme reactive materials. Hence, they may not pass through the column and appear at the detector.
6. The ability of sulphur dioxide to reduce iodine is used in which of the following methods?
a) Colorimetry
b) Conductivitimetry
c) Coulometry
d) Gas chromatography
Answer: c
Explanation: The ability of sulphur dioxide to reduce iodine is used in Coulometry method to measure sulphur di-oxide. The mass of I2 reacted per unit time will indicate the amount of sulphur di-oxide.
7. Which of the following materials are used as electrodes in coulometric method for the measurement of sulphur di-oxide?
a) Gold
b) Silver
c) Platinum
d) Nickel
Answer: c
Explanation: In Coulometric arrangement, the electrodes are made of platinum. These act as the anode and the cathode.
8. Which of the following is the detection limit of coulometric method used for measurement of sulphur di-oxide?
a) 0.1 ppm
b) 1 ppm
c) 2 ppm
d) 0.01 ppm
Answer: d
Explanation: The detection limit of coulometric method is 0.01 ppm. The shift in anode cathode potential is detected by a third electrode.
9. Sensitivity to total sulphur by Flame-photometric detector is which of the following levels?
a) 0.1 ppm
b) 1 ppm
c) 2 ppm
d) 0.01 ppm
Answer: d
Explanation: Sensitivity to total sulphur by Flame-photometric detector is 0.01 ppm. Here, sample air is introduced into a hydrogen-rich air flame.
10. Which of the following methods are not used for the measurement of sulphur di-oxide?
a) Colorimetric method
b) Correlation spectroscopy
c) Paramagnetic analyzers
d) Flame-photometry
Answer: c
Explanation: Sulphur di-oxide cannot be analysed using paramagnetic analyzer. This is because sulphur di-oxide does not have paramagnetic property.
11. Which of the following is the wavelength of the radiation generated when an air stream containing sulphur di-oxide is burned in a hydrogen-rich flame?
a) 243 nm
b) 394 nm
c) 467 nm
d) 516 nm
Answer: b
Explanation: The wavelength of the radiation generated when an air stream containing sulphur di-oxide is burned in a hydrogen-rich flame is 394 mm. A narrow band interference filter is used to shield radiations with all other wavelengths.
12. Given below, is the diagram of Ultraviolet Fluorescence method. Identify the unmarked component.
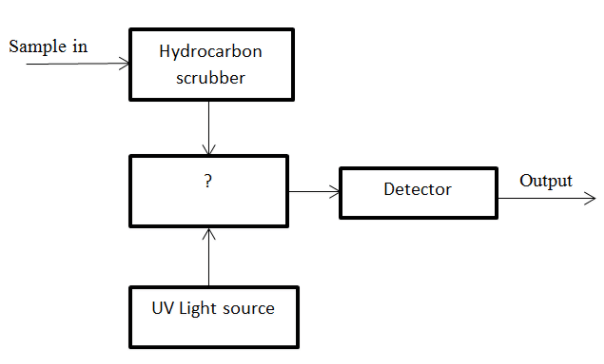
a) Filter
b) Electronic circuit
c) Supply
d) Fluorescence chamber
Answer: d
Explanation: The unmarked block is Fluorescence chamber. Here, fluorescence of SO2 is emitted.
13. In conductivitimetric method, the concentration of sulphur di-oxide is proportional to which of the following parameters of the saw-tooth waveform?
a) Average voltage
b) Peak voltage
c) Slope
d) RMS voltage
Answer: c
Explanation: In conductivitimetric method, the concentration of sulphur di-oxide is proportional to slope of the saw-tooth waveform. Current is recorded every 15 minutes.
14. In conductivitimetric method, to measure the conductivity of the cell, 5V ___________ is applied across the electrodes.
a) DC
b) AC
c) Pulsating DC
d) DC or AC
Answer: b
Explanation: In conductivitimetric method, to measure the conductivity of the cell, 5V alternating current is applied across the electrodes. This is because alternating current avoids polarisation.
15. The response of coulometric method is instantaneous.
a) True
b) False
Answer: b
Explanation: The response of coulometric method is not instantaneous. It may take about 4 minutes for 90 percent of the signal to appear for any concentration of SO2.