HSE and Safety Interview Questions and Answers

What is a BUMP TEST ?
A bump test is a functional test of a gas monitor/detector. Sensors and alarm indicators are tested, ensuring acceptable performance of sensors and monitor before use.
All sensors are challenged with a known quantity of gas, and instrument response is compared to the actual quantity of gas present.
OSHA says, “The International Safety Equipment Association (ISEA), founded in 1933, is a trade association for manufacturers of protective equipment, including environmental monitoring instruments.
The ISEA recommends, at a minimum, verification of sensor accuracy before each day’s use. The only way to guarantee that an instrument will detect gas accurately and reliably is to test it with a known concentration of gas.
Exposing the instrument to a known concentration of test gas will show whether the sensors respond accurately and whether the instrument alarms function properly.
A bump test verifies calibration by exposing the instrument to a known concentration of test gas.
The instrument reading is compared to the actual quantity of gas present (as indicated on the cylinder). If the instrument’s response is within an acceptable tolerance range of the actual concentration, then its calibration is verified. Sensors, especially LEL (combustible) sensors, may be lose sensitivity to the target gas through poisoning.
Silicone compounds including silanes, halogens, halogenated hydrocarbons, sulfur compounds, as well as acids and bases and various VOCs are known poisons. The only way means of identifying loss of sensor sensitivity is by gas-checking or calibration.
As a result, OSHA and instrument manufacturers/end users require a bump test before every day’s use.
What is a Lower Explosive Limit ?
The lower explosive limit (LEL) of a gas or a vapour, is the lowest concentration (in air) that is needed for the gas to ignite and explode.
For example, propane can explode when it reaches 2.1 per cent of the air, by volume. At 2.1%v/v, propane has reached 100% of its lower explosive level. 50% LEL propane is 0.6%v/v.
Most flammable gas detectors measure the percent of LEL present. Some sensors are capable of measuring methane by volume, as well as percent LEL.
There are two explosive limits for any gas or vapor, the lower explosive limit (LEL) and the upper explosive limit (UEL).
At concentrations in air below the LEL there is not enough fuel to continue an explosion; at concentrations above the UEL the fuel has displaced so much air that there is not enough oxygen to begin a reaction.
Concentrations of explosive gases are often given in terms of percent of lower explosive limit (%LEL).
Also Read : Gas Detectors Interview Questions & Answers
What is a Confined Space?
Generally speaking, a confined space is an enclosed or partially enclosed space that:
- is not primarily designed or intended for human occupancy
- has a restricted entrance or exit by way of location, size or means
- can represent a risk for the for the health and safety of anyone who enters, due to one or more of the following factors:
- its design, construction, location or atmosphere
- the materials or substances in it
- work activities being carried out in it, or the
- mechanical, process and safety hazards present
Confined spaces can be below or above ground. Confined spaces can be found in almost any workplace.
A confined space, despite its name, is not necessarily small.
Examples of confined spaces include silos, vats, hoppers, utility vaults, tanks, sewers, pipes, access shafts, truck or rail tank cars, aircraft wings. Ditches and trenches may also be a confined space when access or egress is limited.
Photo-ionization Detector Related Questions
What is a PID?
A photo-ionization detector or PID, uses an electrodeless ultraviolet lamp to ionize chemicals with ionization potentials (I.P) below the range of the lamp and thereby measure their concentrations in parts-per-million (ppm).
A PID is best used to detect low levels (0-2000ppm) of broad band toxics or volatile organic compounds (VOCs).
Sample Site Remediation Procedure using PID: Prior to use, the PID was calibrated against a 100 parts per million (ppm) isobutylene span gas in air matrix. The instrument was then zeroed against the ambient air near the work area.
The PID is useful for qualitative field screening of VOCs and provides a basis for comparison between soil samples collected in the field.
Soil samples were placed into sealable plastic bags and allowed to sit in a warm area for volatilization to occur. Each bag was opened and the tip of the PID was inserted into the head space above each sample.
What are some examples of chemicals that are detected by PIDs?
Acetone, Ammonia, Asphalt fumes, Benzene, Butane, Chlorobenzene, Cyclohexane, Diesel Fuel, Ethyl ether, Ethylene glycol, Formaldehyde, Gasoline, Hexane, Iodine, Isobutylene, Jet fuels, kerosene, Methyl Mercaptan, Mineral Spirits, Nitric Oxide, Octane, Pentane, Propylene, Resorcinol, Styrene, Toluene, Turpentine, Vinyl Chloride, etc.
What are VOCs?
VOCs are Volatile Organic Compounds, organic chemicals that have a high vapor pressure and easily form vapors at normal temperatures and pressure.
The term “organic” indicates that the compounds contain carbon. VOC exposures are often associated with an odour while other times there are no odours. Both can be harmful.
The term is generally applied to organic solvents, certain paint additives, aerosol spray can propellants, fuels (such as gasoline, and kerosene), petroleum distillates, dry cleaning products and many other industrial and consumer products ranging from office supplies to building materials. VOCs are also naturally emitted by a number of plants and trees.
What are the effects of VOC exposure?
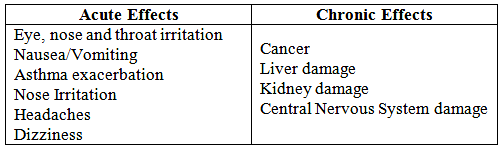
Key signs or symptoms associated with exposure to VOCs include conjunctival irritation, nose and throat discomfort, headache, allergic skin reaction, dyspnea, declines in serum cholinesterase levels, nausea, emesis, epistaxis, fatigue, dizziness.
The ability of organic chemicals to cause health effects varies greatly from those that are highly toxic, to those with no known health effect.
As with other pollutants, the extent and nature of the health effect will depend on many factors including level of exposure and length of time exposed.
Eye and respiratory tract irritation, headaches, dizziness, visual disorders, and memory impairment are among the immediate symptoms that some people have experienced soon after exposure to some organics.
Also Read : Hydrogen Sulfide Gas (H2S)
Toxic Gas Related Questions & Answers
What are TWA, PEL and REL ?
The Threshold Limit Value (TLV(R) ) of a chemical substance establishes the reasonable level to which a worker may be repeatedly exposed, day after day, over a working lifetime without adverse health effects.
TLV(R) is a reserved term of the American Conference of Governmental Industrial Hygienists (ACGIH)(R) . It is however sometimes loosely used to refer to other similar concepts used in occupational health and toxicology. A list of current TLVs(R) and biological exposure indices (BEIs) are published annually by the ACGIH(R) .
A Time Weighted Average (TWA) is a TLV(R) based on a 8-hour workday and a 40-hour workweek. For example the TWA for carbon monoxide is 25 ppm. This means that an average of 25 ppm is considered to be the safe TLV(R) for an 8-hour workday.
A Short Term Exposure Limit (STEL) is a TLV(R) based on a 15 minute average.
A Ceiling is a TLV that should not be exceeded during any part of the work experience.
The Permissible Exposure Limit (PEL or OSHA PEL) is a TLV(R) established by OSHA in the U S for exposure of an employee to a substance or physical agent, and may differ from TLVs(R) in other jurisdictions.
Permissible Exposure Limits are established by the Occupational Safety and Health Administration (OSHA). A PEL is usually given as a time-weighted average (TWA), although some are Short Term Exposure Limits (STEL) or Ceiling Limits.
RELs or Recommended Exposure Limits are TLVs(R) established by NIOSH for exposure of an employee to a substance or physical agent.
Who decides safe levels for toxic gas exposures?
There are various organizations who make recommendations about exposure levels, or threshold limit values (TLVs) for various chemicals. Among them are:
OSHA Occupational Safety and Health Administration. Under the Occupational Safety and Health Act of 1970, the US Congress created OSHA as a Federal agency under the Department of Labor that develops and enforces federal standards for health and safety in the work place. The mission of OSHA is “to assure the safety and health of America’s workers by setting and enforcing standards; providing training, outreach, and education; establishing partnerships; and encouraging continual improvement in workplace safety and health”.
NIOSH National Institute for Occupational Safety and Health. A US Federal agency under the Department of Health and Human Services created by the Occupational Safety and Health Act of 1970, that trains occupational health and safety professionals, and is responsible for conducting research and making recommendations for the prevention of work-related injury and illness.
ACGIH American Conference of Governmental Industrial Hygienists. A voluntary membership organization of professional industrial hygiene personnel in governmental or educational institutions. ACGIH develops and publishes recommended occupational exposure limits each year called Threshold Limit Values (TLV’s) for hundreds of chemicals, physical agents, and includes Biological Exposure Indices (BEI).
Different organizations may not agree on the safe level for different chemical exposures. For example, NIOSH establishes a threshold limit of 35 ppm for a time weighted average (TWA) for carbon monoxide for a normal 8-hour workday and a 40-hour workweek. ACGIH recommends a TWA of 25 ppm.
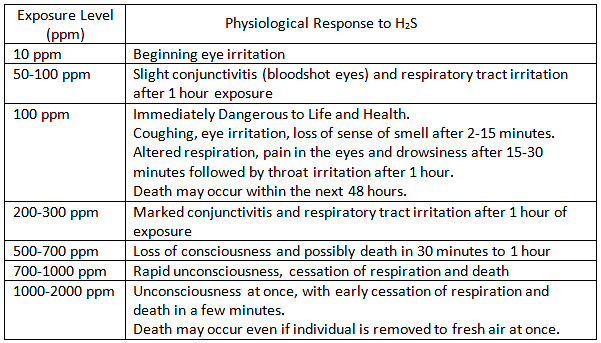
What is H2S?
Hydrogen Sulfide is a colorless, transparent gas with a characteristic rotten-egg odor at low concentrations. It is not detectable by odor at high concentrations. As the level of H2S increases, the sense of smell is lost!
Hydrogen Sulfide is a naturally occurring gas mixed with natural gas or dissolved in the oil or brine and released upon exposure to atmospheric conditions.
ACGIH 2008 recommendations are as follows for Threshold Limit Values for H2S:
A Short Term Exposure Limit (STEL) is a TLV(R) based on a 15 minute average.
Short Term Exposure Level – STEL (15 min Average) – 15ppm
Long Term Exposure Level -TWA (8 hr average) – 10ppm
Also Read : Basics of Gas Monitoring
Carbon Monoxide Related Questions & Answers
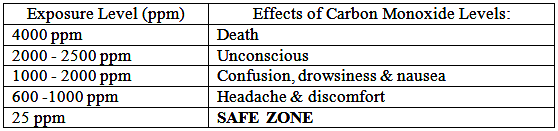
Does everyone react to Carbon Monoxide (CO) in the same way?
The harmful effects of the gas in the air are affected by exposure time and factors such as age, health and size.
Many of us encounter CO regularly and never know it because it is invisible and odorless.
That is why victims of CO poisoning often have no warning that they are in danger – until it is too late.
Symptoms include headache, nausea, chronic fatigue, confusion, and dizziness. Extreme exposure can even cause a coma or death.
What causes Carbon Monoxide (CO) and why is it dangerous?
Carbon Monoxide is a product of incomplete (poor) combustion. It is a direct and cumulative poison.
When combined with blood hemoglobin, CO replaces oxygen in the blood until it completely overcomes the body.
Death from CO occurs suddenly. The victim inhaling the toxic concentration of the gas becomes helpless before realizing that the danger exists.
How much Carbon Monoxide (CO) is safe?
Toxic Gases are measured in ppm (parts per million). 1% volume = 10,000 ppm
Ammonia Related Questions & Answers
What are the hazards of working with Ammonia?
Ammonia is widely used as a refrigerant gas, as well as in the fertilizer industry.
A colourless gas with a sharp, penetrating, intensely irritating odour and a colourless liquid under pressure, it is not considered a flammable gas.
However, a large and intense energy source may cause ignition and/or explosion.
Ammonia gas can decompose at high temperatures forming very flammable hydrogen and toxic nitrogen dioxide. It is a COMPRESSED GAS and a confined space explosion and toxicity hazard.
Ammonia gas is a CORROSIVE GAS and may be fatal if inhaled. It may cause lung injury, and the liquefied gas can cause frostbite and corrosive injury to eyes and skin.
Ammonia gas is a severe respiratory tract irritant. Most people can detect it by smell at 0.6 to 53 ppm. Nose and throat irritation may be noticed at concentrations as low as 24 ppm after 2-6 hours exposure.
A 10-minute exposure to 30 ppm may be faintly irritating to some, while 50 ppm may be found to be moderately irritating by most. A 5-minute exposure to 72 or 134 ppm will cause irritation of the nose and throat for most people.
At 500 ppm, immediate and severe irritation of nose, and throat occurs. Brief exposure to concentrations above 1500 ppm can cause pulmonary edema, a potentially fatal accumulation of fluid in the lungs.
The symptoms of pulmonary edema (tightness in the chest and difficulty breathing) may not develop for 1-24 hours after an exposure.
Numerous cases of fatal ammonia exposure have been reported, but actual exposure levels have not been well documented. If the victim survives, complete recovery may occur depending on the extent of injury to the respiratory tract and lungs.
However, long-term respiratory system and lung disorders have been observed following severe short-term exposures to ammonia.
The TWA established by ACGIH for ammonia is 25 ppm. The STEL is 35 ppm.
Safety Precautions for Ammonia :
- CORROSIVE, COMPRESSED GAS. May also be an EXPLOSION HAZARD, especially in confined spaces. Engineering controls, proper training, protective equipment requirements and personal hygiene measures are essential.
- If ammonia is released, immediately put on a suitable respirator and leave the area until the severity of the release is determined. In case of leaks or spills, escape-type respiratory protective equipment should be available in the work area.
- Never work alone with this chemical.
- Unprotected persons should avoid all contact with this chemical, including contaminated equipment.
- Use a closed handling systems for processes involving this material. If a closed handling system is not possible, use the smallest possible amounts in a well-ventilated area separate from the storage area. Prevent the release of gas into the workplace air. For large-scale operations, consider the installation of a leak detection system with an alarm.
- Do not use with incompatible materials such as oxidizing agents (e.g., nitrogen oxide), halogens (e.g., chlorine, fluorine) and heavy metals (e.g., mercury, silver).
- Regularly inspect cylinders for corrosion or other damage or leaks before handling. Use corrosion-resistant transfer equipment.
- For large-scale handling operations use non-sparking ventilation systems, approved explosion-proof equipment and intrinsically safe electrical systems in areas of use. Keep aisles and exits free of obstruction. Avoid all ignition sources (e.g., sparks, open flames, hot surfaces). Do not use near welding operations. Keep away from heat. Post “NO SMOKING” signs. It is very important to keep areas where this material is used clear of other materials which can burn (e.g., cardboard, sawdust).
- Never perform any welding, cutting, soldering, drilling or other hot work on an empty vessel, container or piping until all the ammonia has been cleared. Do not heat compressed gas cylinders. Do not handle cylinders with oily hands. Leave cylinder cap on cylinder until cylinder is secured and ready for use. Always secure cylinders to a wall, rack or other solid structure in an upright position.
- Use the appropriate pressure regulator. Before connecting the cylinder for use, make sure that back feed from the system into the cylinder is prevented.
- Do not open a damaged cylinder. Open cylinder valve slowly to prevent rapid decompression and damage to valve seat. Keep cylinder valves clean and free from contaminants (particularly oil and water). Make sure valves on gas cylinders are fully opened when gas is used. Open and shut valves at least once a day, while cylinder is in use, to avoid valve ‘freezing’.
- Shut flow off at cylinder valve and not just at the regulator after use. Replace outlet caps or plugs and cylinder caps as soon as cylinder is disconnected from equipment.
- Make sure cylinders are labeled clearly. Avoid damaging cylinders. Move cylinders by hand truck or cart designed for that purpose.
- Keep empty cylinders under slightly positive pressure. Do not use cylinders as rollers or for any other purpose than to contain the gas as supplied.
- Follow handling precautions on Material Safety Data Sheet. Have suitable emergency equipment for fires, spills and leaks readily available. Practice good housekeeping. Maintain handling equipment. Comply with applicable regulations.
Also Read : Interview Questions on Smoke detection system
Really helpful site for EHS guys.