Ozone measurement
Ozone (O3) is an instable molecule of three oxygen atoms and a very strong oxidizing agent. At room temperature it is a gas. Due to its instability it cannot be stored in pressurised cylinders and has to be prepared on site.
O3 is an eco-friendly disinfectant. However, its great disinfection strength can only be used to good advantage in suitable reactors with a reaction time of at least 3 minutes. The long-term effect of O3 is only a few minutes.
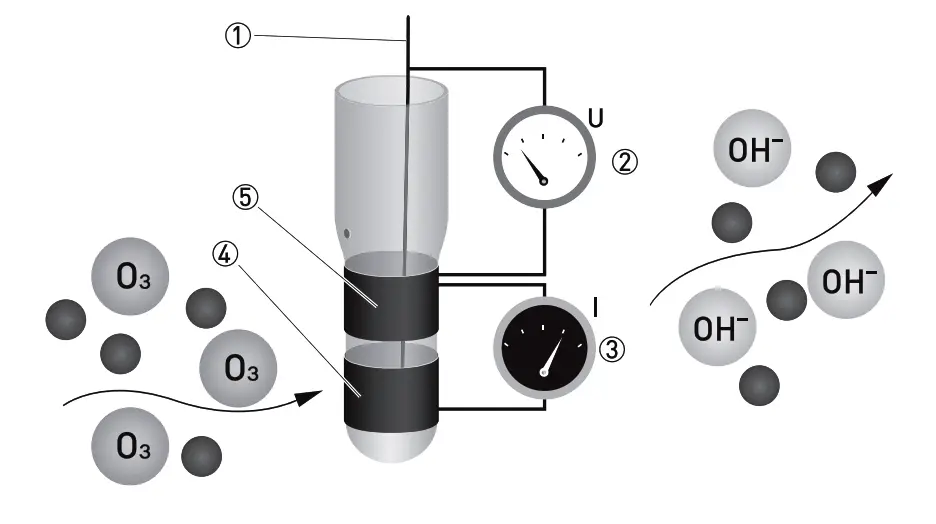
Image Credits : krohne
- Reference electrode
- Applied ozone specific potential
- Current needed to maintain the constant potential
- Counter electrode
- Measuring electrode
O3 is measured potentiostatic with measuring and counter electrodes of pure gold and an Ag/AgCl reference. The measurement shows high selectivity towards ozone. A precise potential is built up between the measuring and the reference electrode. The measuring electrode starts polarising, i.e. ions collect close to the electrode to neutralize the electrical field. O3 molecules that hit the surface take a defined portion of the charge with them.
The controller measures the potential between measuring and reference electrode and readjusts the charge on the electrode surface. The current needed to maintain a constant potential is directly correlated to the dissovled ozone concentration in the measuring medium. The sensor design with 3 electrodes in a single rod enables us to use our patented cleaning procedure ASR (patent Dr. A. Kuntze) providing you with a low-maintenance measuring setup.
Source : krohne