H2S Analyzers Questions & Answers
1. Which of the following is not an advantage of electrochemical cells used for analysis of H2S?
a) Portable battery operated instrument
b) Pocket sized instrument
c) No pumps are needed
d) No interference from background gases
Answer: d
Explanation: Electrochemical cells are affected by interference from background gases. Some of them are sulphur dioxide, carbon monoxide, nitric oxide and chlorine.
2. Which of the following methods are mainly used in combustible gas detection equipment?
a) Lead acetate tape staining method
b) Solid state sensor
c) Gold film sensor
d) Electrochemical cells
Answer: b
Explanation: Solid state sensors are mainly used in combustible gas detection equipment. It is a semiconductor sensor.
3. Which of the following is not an advantage of solid state sensor used for analysis of H2S?
a) No sampling system
b) It can be used in conditions involving vibration
c) It can be used in corrosive atmosphere
d) No interference from background gases
Answer: d
Explanation: Solid state sensors are affected by interference from background gases. Some of them are hydrogen, isopropanol and ethyl and methyl mercaptan.
4. Which is the compound that forms the stain in Lead Acetate Tape Staining method used for the analysis of Hydrogen Sulphide and what is the colour of the stain?
a) Lead Sulphide, red colour
b) Lead Sulphide, brown colour
c) Hydrogen acetate, white colour
d) Hydrogen acetate, green colour
Answer: b
Explanation: Hydrogen Sulphide reacts with Lead Acetate to form Lead Sulphide which is brown in colour. Hydrogen acetate in other words is acetic acid and is colourless.
5. Which of the following methods is not used for detection of hydrogen sulphide?
a) Lead acetate tape staining method
b) Solid state sensor
c) Chemiluminescence method
d) Electrochemical cells
Answer: c
Explanation: Chemiluminescence method cannot be used for detection of hydrogen sulphide. Others method that are used are using photometric analyser and using gold film sensor.
6. In solid state sensor, when heated to 150o to 300o which of the following occurs?
a) Resistance decreases with increase in H2S concentration
b) Resistance increases with H2S concentration
c) No change in resistance occurs
d) Sensor does not respond in this temperature range
Answer: a
Explanation: In solid state sensor, when heated to 150o to 300o resistance decreases with increase in H2S concentration. The decrease in resistance is a logarithmic function of H2S concentration.
7. In gold film sensor, the change in resistance is proportional to concentration of H2S.
a) True
b) False
Answer: a
Explanation: Gold film sensor works on the principle of absorption. The change in resistance is
proportional to concentration of H2S.
8. Which of the following occurs in Electrochemical cells used for detection of hydrogen sulphide?
a) Change in resistance
b) Redox reaction
c) Oxidation-reduction reaction
d) Change in colour
Answer: c
Explanation: In Electrochemical cells, an oxidation-reduction reaction occurs in the presence of hydrogen sulphide. A current starts flowing and it is proportional to the concentration of hydrogen sulphide.
9. Which of the following is not a component of solid state sensor used for detection of hydrogen sulphide?
a) Heater
b) Thermistor
c) Semiconductor film
d) Photo detector
Answer: d
Explanation: For measuring H2S, the sensor must be maintained at a particular temperature. This is done by using heater. The temperature is monitored using thermistor.
10. The diagram of Electrochemical cell used for the detection of hydrogen sulphide is given below. Identify the unmarked component.
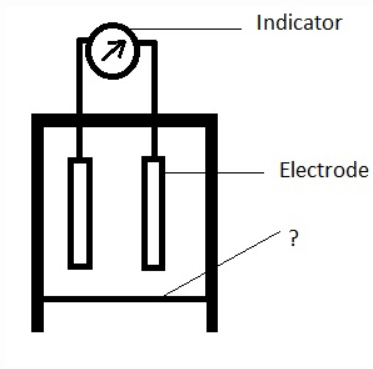
a) Filter
b) Gas permeable membrane
c) Elastic layer
d) Electrolyte
Answer: b
Explanation: The unmarked component is gas permeable membrane. Electrochemical cell is an electrochemical gas diffusion sensor.