The anode and cathode are the two most important parts of an electrical or electrochemical system. The anode and cathode are like two sides of a coin. Both exist together and make a system complete. Anode and cathode mostly come into the picture when we talk of batteries, diodes, and other components having electrodes.
Today in this article we will have a lookup at both the anode and the cathode. We will learn the differences between the anode and cathode.
What is Anode?
Anode is one of the electrodes of a device like a battery or a diode, at which oxidation takes place. Oxidation is nothing but the process by which electrons leave a surface. Hence, on the anode, we can see the electron loss.
In other words, we can say that the electrode from which electrons leave is an anode.
What is Cathode?
Cathode is the second electrode of a device like a battery or a diode, at which reduction takes place. Reduction is the process in which the electrons enter a surface. Hence, on the cathode, we can see the electron come into that device or electrons are received.
In other words, we can say that the electrode in which electrons enter is a cathode.
Difference between Anode and Cathode
Let us see some of the key differences between Anode and Cathode:
| Anode | Cathode |
| The electrode on which the oxidation reaction occurs is an anode | The electrode on which the reduction reaction occurs is a cathode |
| Anode is the positively charged electrode | Cathode is the negatively charged electrode |
| Anode donates the electrons | Cathode accepts the electrons |
| In an electrolytic cell, the oxidation reaction will place at the anode. | In an electrolytic cell, the reduction reaction will place at the cathode. |
| In a galvanic cell, the reduction reaction will place at the anode. | In a galvanic cell, the oxidation reaction will place at the cathode. |
| Anode is made up of material like graphite | Cathode is made up of material like is lithium cobalt oxide |
If we take a real-life example then consider a chemical reaction of electrolysis of NaCl.
In the electrolysis of NaCl (commonly known as salt) chemical, when we insert two electrodes in NaCl and water solution, the electricity passes when we connect a battery as shown in the below figure.
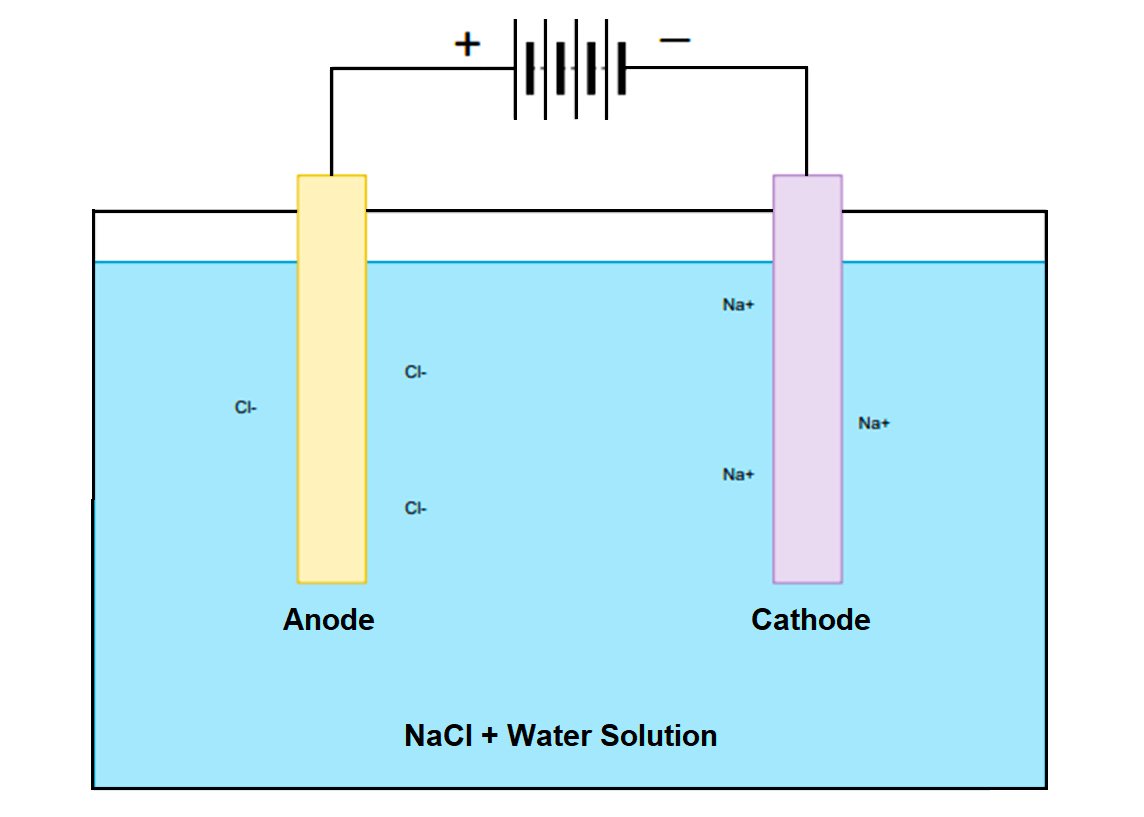
The anode is connected to the positive terminal of the battery and hence the anode will start developing a positive charge. Hence, Cl- ions present in the NaCl + H2O solution will move toward the anode.
The cathode is connected to the negative terminal of the battery and hence the cathode will start developing a negative charge. Hence, Na+ ions present in the NaCl + H2O solution will move toward the cathode.
Hence, at the cathode, we can see deposition of the sodium metal, and on the anode, bubbles of the chlorine gases form.