Read about frequently asked Dissolved Oxygen Analyzer Interview Questions & Answers useful to get job placement as a Analyzer Technician or Engineer.
Dissolved Oxygen Analyzer
What is dissolved oxygen ?
Dissolved oxygen refers to oxygen dissolved in water. Its concentration is expressed as the amount of oxygen per unit volume and the unit is mg/L.
Biologically, oxygen is an essential element for respiration of underwater life and also acts as a chemical oxidizer. The solubility of oxygen in water is affected by water temperature, salinity, barometric pressure, etc. and decreases as water temperature rises.
Dissolved Oxygen Sensor Working Principle ?
The measurement of dissolved oxygen is based on the well-known Clark cell principle. An oxygen-permeable membrane isolates the electrodes from the sample water, thus obviating the need for sample conditioning. Other reducible or oxidizable ions do not interfere, because they cannot pass through the gas-permeable membrane.
A constant voltage supply powers two electrodes, maintaining each at a constant potential. A gold, working electrode (cathode) reduces the dissolved oxygen to hydroxyl ions:
O2 + 2H2O + 4e- => 4OH-
A large silver counter-electrode (anode) provides the oxidation reaction that occurs on its surface:
4Ag+ + 4Br – => 4AgBr + 4e-
The reduction of oxygen is the current limiting reaction, thus making the cell current linearly proportional to the dissolved oxygen concentration.
Electrochemical reactions and diffusion rates are temperature-sensitive. The measuring cell, therefore, is equipped with a temperature sensor which allows for automatic temperature compensation.
Temperature compensation of solution being measured ?
The permeability of the membrane used for the membrane electrodes changes exponentially with temperature.
The amount of saturated dissolved oxygen also changes exponentially with the temperature of the water being measured. Thus, a temperature sensor is incorporated into the senor to perform automatic temperature compensation.
Salinity compensation of solution being measured ?
If the salinity concentration increases under the same conditions of temperature and atmospheric pressure, the amount of dissolved oxygen decreases.
Thus, to measure water having a high salinity concentration, it is necessary to set a saturated dissolved oxygen value for the relevant salinity concentration upon calibration.
Barometric air pressure compensation
The concentration of dissolved oxygen is affected by air pressure differences due to weather conditions or altitude.
Dissolved oxygen analyzers uses a barometric sensor built into the converter, which compensates for the effects of atmospheric pressure ranging from 900 to 1100 hPa. The barometric air pressure compensation is related to percent saturation.
When we do Calibration of the dissolved oxygen analyzers
Calibration of the dissolved oxygen analyzer is performed in the following situations:
| – | When a new dissolved oxygen sensor is installed |
| – | When the membrane assembly is replaced and/or the electrolyte solution is replaced |
| – | When the sensor has been disassembled and reassembled for maintenance |
| – | When a measuring error after cleaning exceeds the acceptable deviation from the reference method |
How does the Clark dissolved oxygen probe work?
The Clark cell discovered by Dr. Clark in 1956 is an ampherometric cell that is polarised around 800 mV. Reduction of oxygen is achieved between 400 to 1200 mV.
Hence the need for a voltage of around 800 mV. In the Clark cell this is provided externally by a battery source. The Clark cell is built around the popular Ag/AgCl half-cell and a noble metal such as gold.
What parameter is being measured by the dissolved oxygen content of a solution?
The dissolved oxygen probe meters does not measure the actual amount of oxygen in water, but instead measures the partial pressure of oxygen in the water sample.
Oxygen’s partial pressure is dependent on both the salinity and temperature of the sample.
How does temperature affect dissolved oxygen measurements?
The temperature of a water sample has an inverse relationship to the solubility of oxygen. As shown below, an increase in temperature reduces oxygen content.
This relationship is non-linear and has been established empirically.
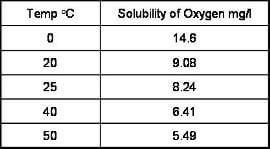
How does atmospheric pressure affect dissolved oxygen measurements?
Due to the fact that the oxygen pressure reduces at higher altitude, the solubility of oxygen reduces with increase in altitude (or reduction of atmospheric pressure).
How does salinity affect dissolved oxygen measurements?
Salinity has an inverse relationship to oxygen solubility. As the salinity of the solution increases, the solubility of oxygen reduces. Results in mg/l.
Why is there a “warm-up time” with dissolved oxygen probes and how long is it?
In Clark cells, an external polarising voltage of approximately 800 mV is applied to the electrode.
When the probe is disconnected, the power supply is cut off. On connecting the probe again, the user must wait for the probe to be polarised i.e. for the current loop to be stabilised. This warm up time is approximately 10 minutes.
How can dissolved oxygen be measured using fluorescence?
A very specific energy wavelength is transmitted to a ruthenium compound immobilized in a sol-gel matrix. The ruthenium will absorb this energy, changing the outer electron’s energy level.
The electron will then collapse back to its original energy state, emitting the energy as a photon with a different specific wavelength. This is called “fluorescing”. If the intensity of the transmitted wavelength is tightly controlled, the amount of fluorescing is both predictable and repeatable.
If oxygen molecules are present the amount of fluorescing is reduced, referred to as “fluorescence quenching”. By measuring the amount of quenching it is possible to determine the amount of oxygen present.
What is the difference between fluorescence and luminescence?
While the two methods are similar, there is one key difference. Fluorescence, which is the method used by Insite, is the measurement of the immediate reaction of a material in response to an excitation energy source.
Luminescence is the measurement of the time it takes the material to recover after the excitation energy source is removed. This method is the one in use by all other manufacturers currently marketing “optical” type DO systems.
APPLICATIONS OF DO ANALYZER :
NATURAL WATER
To ensure the state of dynamic equilibrium of DO maintained by biochemical deplection by bacteria for the food chain and continued re oxy-generation by aeration and photosynthesis and thus the availability of right amount of DO which is vitally important for flora and fauna in water.
To detect pollution – lower level of DO (normal 7.1 ppm) will adversely affect the aquatic life.
SEWAGE WASTES
To ensure biochemical break-down treatment of sewage which is achieved by bacterial attack which requires DO.Non-availability of DO leads to increase activity of anaerobic, sulphate reducing bacteria resulting in production of hydrogen sulphide causing serious corrosion problem.
MICROBIAL STUDIES
To study effects of drugs on the brain tissues, other organs/limbs, circulation system muscle and connective tissues, malignant tissues and surface.
AGRICULTURE
To ensure sufficient aeration in soil needed for underground respiration of plant roots in addition to photo synthesis.
FOODS & DRUGS
To ensure oxidation is not adversely affecting the food stuffs. To ensure DO requirements for fermentation in foods, distilleries and Bio-synthetic preparations in Drugs & Chemicals.
BOILER FEED WATER
To ensure proper level of DO to avoid oxidative corrosion of Boiler pipes in steam generation, heaters etc.

I understand, a question, other gases disolved in the liquid could afect the mesurament or the disolved oxygen concetration per se ?
other question, why the unit of mesure is in mg/l and not in noles/l?
Using an spectrofluorimeter a molecule called the fluorophore , had a fluorescence enision that is dependent on the oxygen concentration.
Whit the some principle is also posible mesure the pH whit a glass electrode permeable to hydrogenions.
Excuse my english, my natural languaje is spanish and y an dislexic too, best regards.
Kyp Durron